PORTAL
The Participant’s Portal link on the UK NEQAS for H&I website or type https://ukneqashandi.naqoda.cloud/ into your internet browser. The site is available on any device including tablets and smartphones.
UK NEQAS for H&I have provided a number of user guides and quick guides which are available on the NEQAS website and as links at the footer of the Portal site to assist users in navigating the system. The office is also happy to take phone calls on +44 (0)1443 622185 and emails at UKNEQASHandI@Wales.NHS.UK. We always welcome your feedback on any issues so we can direct ongoing improvements.
To make any changes to staff you need to be registered as a Primary User. Primary Users can simply click on the Staff option in the menu bar. This is bring up a list of all users for you lab and you can add, delete or change staff roles as required.
Click on Staff from the top menu. The ‘Lab Staff’ page allows you to view/add/remove staff as users of the UK NEQAS for H&I Participant Portal.
If you need any assistance please contact the NEQAS office at UKNEQASHandI@Wales.NHS.UK.
The Portal is automated to send result reminders to those participants who have not submitted results. The system sends out an email per sample, not per scheme so it is possible that users may get multiple notices. The system was designed this way as samples are entered on an individual basis for each scheme.
Several different user access levels have been created to allow laboratories to assign
different user roles to staff members using the system:
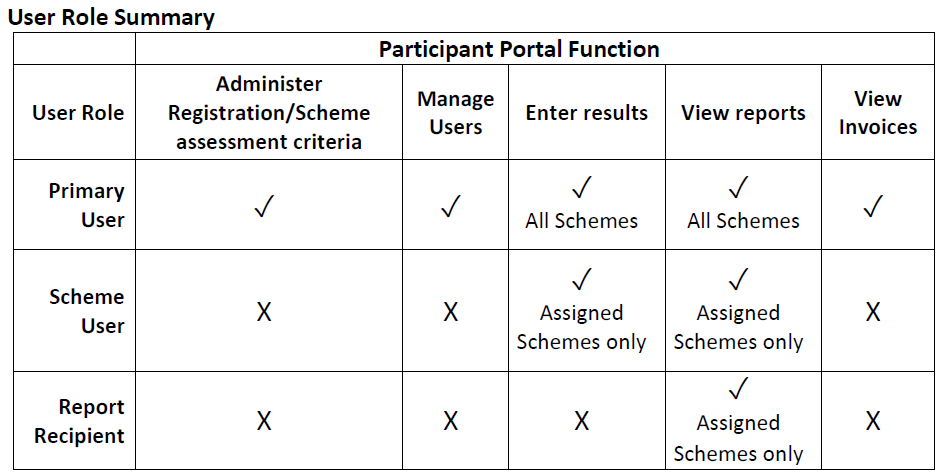
There is no limit to the number of users that can be added. Users of the system will be able to see relevant notices and receive emails relating to EQA scheme operation.
If you forget your password, please use the forgotten password link.
- Click on Lost your login data on the login page
- Enter your email address and click Send
You will now receive an email from no-reply.ukneqashandi@welsh-blood.org.uk with a link to reset your password. Please be patient as this email may not come through immediately and may go to a spam/junk folder so please check these folders.
The link in the email will take you to a page which will show a notification informing that your password has expired. Please create a new password. You should now click login to access the system with your email address and new password.
The My Lab menu displays the general contact details of the laboratory. The name of your lab, as assigned by UK NEQAS for H&I, is displayed here.
The lab number (Lab ID) is also displayed on this page under ‘Reference’.
REGISTRATION
Laboratories that are not currently registered for any UK NEQAS for H&I Schemes can
register their interest to participate using the system.
On the Participant Portal home page click on New Lab Registration and complete the information requested:
Once your application for registration in UK NEQAS for H&I Schemes has been accepted, you will be sent an e-mail with your login details. You will need to access the system to complete the registration process.
Participation is for one year (April to March) after which time you will be asked to confirm your re-registration.
Primary Users can amend the lab registration at any point in the year by clicking the appropriate year in the Annual Registration section. This will open up the registration screens where you can view and amend what Schemes your lab will participate in. If you add an additional Scheme part way through the year you will only be billed for upcoming samples.
Any time after initial registration is complete, a certificate of registration and scheme fee quotation can be produced.
- Click Registration > Scheme Registration
- On the row for the relevant distribution year, click the drop down arrow and select ‘Certificate’ or ‘Quote’ as required.
Given that there is only one ‘correct’ result for each test, we recommend that laboratories do not have two separate registrations. We recommend that you subject one analyser to EQA and use daily IQC to assure that both are giving the same results. Alternatively, where the two analysers employ different processes, the EQA exercises can be alternated between the two, or half the samples tested on the first analyser and the other half on the second.
SAMPLES
Samples are sent over the course of a financial year (April to March) according to set dates. The number and frequency of samples sent for each scheme are detailed in the Schemes Menu.
The type of delivery option you have selected will affect the precise delivery date. Please contact the NEQAS office on UKNEQASHandI@Wales.NHS.UK if you would like to discuss the options available and what would best suit your laboratory.
For the majority of UK NEQAS for H&I Schemes all material is distributed to participants, therefore repeat samples are not available.
A limited supply of serum and DNA samples are retained by UK NEQAS for H&I, which may be available to participants upon request.
It may be possible to request an extra set of samples prior to a distribution date. Please contact the NEQAS Office on UKNEQASHandI@Wales.NHS.UK to confirm.
Please contact the NEQAS office on UKNEQASHandI@Wales.NHS.UK. If you have selected delivery by courier or international post we will be able to tract your package.
Participants that have been unable to test samples MUST state log on to the Portal result submission pages and enter a reason for not testing. Technical issues and invalid results (e.g. control failures, replicate issues, sample quality issues) should be reported as ‘Not Tested’ with the reason stated. ‘Not tested’ reports will not be assessed.
Participants can view a result summary screen in the Portal detailing which user entered and verified the results for each sample.
Select Results then All Results and then use the drop down option to select a summary.
UK NEQAS for H&I schemes are designed to be a continuous evaluation of laboratory test results. We appreciate that delivery costs can be expensive, but the schemes need to monitor laboratory performance on more than 1 occasion to give confidence in the results for patients/donors.
RESULTS
The system is designed to allow results to be entered, review and submitted by a single or multiple users, depending how your laboratory wishes to submit EQA results.
To remove the risk of data entry errors we recommend that one user enters the results, then another user can access the system to review and submit the results.
Submitted results may be edited and resubmitted anytime up to the result deadline. This can only be performed by a primary user.
Anonymised performance tables are used to view results and method information reported by all participants.
- Click on Reports and Performance Tables to access the results summaries.
The closing date determines the timing of each cycle for submission. The closing date for submission is clearly stated on the assessment run delivery letter. This form also advises that submissions cannot be accepted after this date.
In some instances, submissions received after this date may be accepted at the discretion of the Scheme Manager. This is providing the participant states a genuine reason for the late submission of material.
Participants that have been unable to test samples MUST state log on to the Portal result submission pages and enter a reason for not testing. Technical issues and invalid results (e.g. control failures, replicate issues, sample quality issues) should be reported as ‘Not Tested’ with the reason stated. ‘Not tested’ reports will not be assessed. If you do not state a valid reason for not testing samples your lab will be penalised.
All participants are expected to return results promptly within the specified reporting period. Participants who fail to return results or return results after the distribution closing date may not be assessed.
PERFORMANCE
Notification will be sent when your report is available to view in the Participant Portal.
- Click on Reports and Performance Reports to access all laboratory reports.
The table will display a list of available Scheme Assessment Reports and Annual Performance Reports. Unsatisfactory performance and close-out letters will also appear in this list.
If you need any advice or assistance please contact the NEQAS office at UKNEQASHandI@Wales.NHS.UK. You can also call the office directly on +44 (0)1443 622185.
If your laboratory fails to meet the Schemes’ minimum performance criteria (link), your laboratory will receive notification that you are an unsatisfactory performer.
You should complete root cause analysis to find the cause of the error and complete a Corrective and Preventative Action (CAPA) form within 20 days of the notification. More information is available in the Unsatisfactory Performance pages.
An appeal against an assessment, including any relevant laboratory evidence, should be made in writing to ukneqashandi@wales.nhs.uk. Any errors made by UK NEQAS in assigning your results will be rectified and an amended report issued.
Appeals are reviewed on an individual basis by the Directors and Operations Manager in the first instance and may be discussed at Steering Committee meetings.
UK NEQAS for H&I has a policy in place to deal with complaints from participants. Complaints will only be treated as such if the participant states clearly that they are making an official complaint. Complaints should be in the form of an email or letter.
All complaints will receive written acknowledgement within 2 working days and receive a prompt and thorough investigation, to identify the root cause of the problem. All formal complaints will be discussed with the Steering Committee.
Complaints aim to be resolved with a final written response within 30 days. However, if this is not possible, an estimated close out date will be supplied and regular communication sent with updates to the progress of the complaint. Any unresolved complaints can be directed to the Chair of the Steering Committee, the Chair of the National Quality Assurance Advisory Panel for Immunology (NQAAP) or the Royal College of Pathologists’ Joint Working Group for Quality Assessment in Pathology (JWG).
At all times during the complaints procedure, participant confidentiality will be maintained.
Complaints about UK NEQAS for H&I should be made in writing to:
Amy De’Ath (Operations Manager)
UK NEQAS for H&I, Welsh Blood Service,
Ely Valley Road,
Talbot Green,
Pontyclun
CF72 9WB.
Tel: 01443 622185
E-mail: ukneqashandi@wales.nhs.uk.
Yes. The anonymity of each participating laboratory will be maintained at all times unless you are a UK based laboratory deemed as a persistent poor performer. The laboratory identity will only then be disclosed to the Chair of the NQAAP for Immunology panel. For further information click here
Data from participants is treated with strict confidentiality. Each laboratory is registered under a unique laboratory number, which is only known to the Schemes’ Manager and UK NEQAS for H&I staff. Laboratory identifiers and performance information are confidential and will not be released to a third party without the written permission of the Head of the participating laboratory. Click here to view the UK NEQAS for H&I Privacy Disclaimer.
FEES AND INVOICING
Any time after initial registration is complete, a scheme fee quotation can be produced.
- Click Registration > Scheme Registration
- On the row for the relevant distribution year, click the drop down arrow and select ‘Quote’.
Primary users can access invoices for EQA participation. Your official invoice from UK NEQAS for H&I (issued by our host Velindre University NHS Trust) can be accessed in the Portal by clicking Invoices from the top menu.
UK NEQAS for H&I Team
If you need any assistance please contact the NEQAS office at UKNEQASHandI@Wales.NHS.UK. You can also call the office directly on +44 (0)1443 622185.
The Steering Committee meets three times a year and is made up of a panel of experts in the field of H&I with the specific aim to advise on the design and running of existing or new schemes, provide opinions on any appeals raised by participants, and provide expert knowledge to assist unsatisfactory performers (if required).
